Frequently Asked Questions
This document is a companion to “Characterizing the Geothermal Lithium Resource at the Salton Sea,” a technical report funded by the U.S. Department of Energy. The project was a one-year pilot study conducted by researchers at Lawrence Berkeley National Lab, University of California Riverside, University of California Davis, Geologica, MIT, and University of Auckland. The goal of the study was to evaluate resources in the Salton Sea geothermal field and to identify the potential environmental impacts of expanding geothermal production and extracting lithium. We created this FAQ document to make the information in the technical reports more accessible.
Since the Lithium Valley Commission convened in September 2020, community members have shown up to share their perspectives about the lithium resources, the extraction process, and the environmental impact. We identified high-priority questions by analyzing transcripts of Lithium Valley Commission meetings, pulling all the questions that were submitted to the public comment docket or asked during community and tribal workshops, and consulting with local community advocacy organizations.[1] The result is the list of questions contained in this document, with responses from our study and other references. We can’t answer all of the questions, either because we don’t have enough data yet or because it’s outside our scope—for example, our expertise is in geology and environmental sciences, so we do not address social impacts. When we aren’t able to provide answers, we explain why and point to other sources of information.
A lot of the information in the technical report is about geothermal energy, and that’s because those facilities have been operating for decades and are required to submit data about their performance to the state of California. Consequently, we have data about their emissions, how much waste they generate, and even the composition of the brine. The fact that so much data about the geothermal facilities exists demonstrates the value of strong environmental protection laws; with continued access to this type of information as an industry develops, we can verify that companies are operating safely and sustainably. We provide links to databases about geothermal facilities in this document to help people understand how they can access the information we used.
By contrast, no lithium extraction facilities are currently in commercial operation, so we can’t observe the same type of data about lithium extraction. However, each company’s proprietary technology, currently in development, is based on established chemical processes. Scientists at Berkeley Lab and the University of California Riverside, who are familiar with these processes and the various technologies companies could use, have made educated assumptions about the inputs and outputs of the eventual lithium extraction and refining process. These scientists also based their assumptions on information from companies’ environmental impact reports.
The first section of this document gives background information on lithium and geothermal, along with a guide to units that are commonly used in our report. The next section talks about lithium resources in the Salton Sea Geothermal Field and the lithium extraction process. The third section is about water use – both how much water could be used, and where it will come from. The fourth section is about other environmental impacts, like air emissions and waste. Finally, we address topics that were outside the scope of our study and, where possible, point readers to other sources where they can learn more.
[1] You can read more about these efforts in the Community Engagement chapter of the technical report.
Background
Lithium (abbreviated “Li”) is a light, silvery metal that is used to make batteries, ceramics, and medications. It is found in certain types of rock (known as “granite pegmatite”), in claystones, and in salty water called brine. There is a lot of focus on lithium right now because it is used to make batteries for electric vehicles (EVs). California is one of many places around the world that is replacing fossil fuel cars with EVs because they reduce greenhouse gas emissions and local pollution. Lithium batteries also power devices (e.g., computers and smartphones) and are increasingly being used to store energy on the electricity grid. As a result, demand for lithium is growing significantly.
Most lithium is mined in Chile, Argentina, or Australia. The U.S. government is funding research about potential sources of lithium inside the country so we don’t have to rely as much on importing it from other countries.
(References: International Energy Agency, 2021; Jaskula, 2023.)
The word “geothermal” is a combination of the words for Earth (“geo”) and heat (“thermal”). Geothermal power plants can only operate in parts of the world where there are natural hot spots underground, and they use that heat to produce electricity.
There are different ways to produce geothermal energy. The power plants near the Salton Sea use a technology called “flash steam plants.” In these plants, brine (the hot salty water mentioned above) from the underground reservoir is brought to the surface through a very large pipe. The brine is extremely hot (nearly 600° Fahrenheit) and has a very high pressure. The brine goes through a “separator,” which separates steam from the brine. The steam is used to produce electricity. This part of the operation is similar to conventional power plant, but since geothermal plants don’t need to burn natural gas or coal to generate steam, they have far fewer emissions.
The brine that hasn’t been turned into steam is reinjected back into the reservoir to maintain the pressure underground. Before the brine gets reinjected, there is an opportunity to recover some minerals from it. This is when the lithium extraction process, known as “direct lithium extraction” (DLE), could take place.

John L. Featherstone Powerplant
Photo Credit: Jeremy Snyder, LBNL
Metric ton (MT): One metric ton is 1,000 kilograms, or approximately 2,204 pounds.
Lithium Carbonate Equivalent (LCE): Quantities of lithium are often reported in terms of LCE, which stands for lithium carbonate equivalent. Expressing quantities in terms of LCE makes it easier to compare between different lithium compounds, particularly lithium carbonate and lithium hydroxide, which can both be used to make batteries. One metric ton (MT) of Li metal is enough to make 5.32 MT of LCE. One MT of LCE is enough to produce batteries for approximately 22 Teslas, 45 Fiat 500, or over 2,500 e-bikes.[1]
Acre-feet (AF): One acre-foot is the amount of water needed to cover one acre of land to a depth of one foot. It’s a way to measure large quantities of water and is used in most discussions of water use in Imperial Valley. One AF is equal to 325,851 gallons, about as much as one-half of an Olympic-sized swimming pool.
Megawatt (MW): A megawatt is unit of power, and power is the rate at which energy is produced. One MW is enough to power approximately 750 homes at once. In the context of this report, MW is usually used in reference to the generating capacity of a geothermal power plant. For example, a 50 MW geothermal power plant can power roughly 37,500 homes.
[1] Assumptions: 85 kWh Tesla battery, 42 kWh Fiat 500 battery, 0.75 kWh e-bike battery, with composition of .1kg Li/kWh (based on “BatPaC 5.0” default material composition values). BatPaC is software from Argonne National Laboratory that estimates the costs of manufacturing lithium-ion batteries; see https://www.anl.gov/partnerships/batpac-battery-manufacturing-cost-estimation.
A final introductory note about terminology. Several terms are used to describe the geothermal and lithium resource near the Salton Sea, and this can be confusing. All terms refer to the area that contains the underground reservoir of hot brine, but there are subtle differences.
The “Salton Sea Known Geothermal Resource Area” (SSKGRA) refers to a boundary that was made by the U.S. Geological Survey in the 1970s. There are dozens of designated KGRAs across the U.S. Land is classified as a KGRA if “the prospects for extraction of geothermal steam or associated geothermal resources from an area are good enough to warrant expenditures of money for that purpose” (Godwin et al., 1971).
The “Salton Sea Geothermal Reservoir” (SSGR) refers to the entire underground system containing brine, heat, lithium, and other minerals. The exact boundaries of the system are not fully known.
The “Salton Sea Geothermal Field” (SSGF) is the subset of the system where the existing geothermal power plants are located.
“Lithium Valley” is more of a concept than a physically defined place. The California Energy Commission’s Blue Ribbon Commission on Lithium Extraction defined Lithium Valley as “a world-class lithium industry in California centered on recovery of lithium from geothermal brine in the Salton Sea region and the expansion of geothermal energy production, along with creating direct and related economic and community development opportunities.” Imperial County is in the process of developing a specific plan for Lithium Valley, using the boundaries shown in the map to the below.
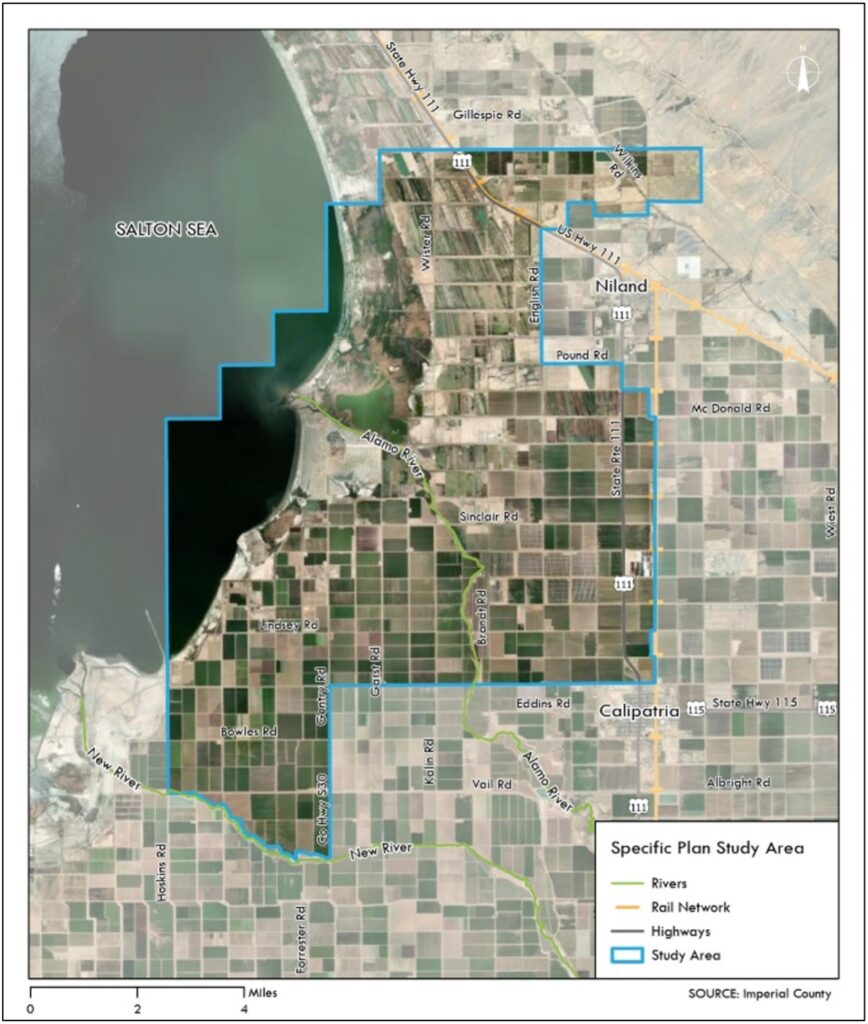
Specific Plan Study Area as presented by Imperial County.

Map of the Salton Sea region displaying the SS-KGRA boundaries, geothermal powerplants, nearby communities, and Native Lands boundaries
Lithium Resources in the Salton Sea Region
The deposits are concentrated around the southeast shore of the Salton Sea, in a zone called the Salton Sea Known Geothermal Resource Area (SSKGRA). The lithium is not in the Salton Sea, but rather dissolved in a nearby reservoir of brine that is distributed throughout the bedrock about a mile below the Earth’s surface. The SSKGRA has that name simply because of its close proximity to the Salton Sea, so the Salton Sea was used as a reference point. For example, there is another nearby resource area called the Brawley Geothermal Field that is located near (but not in) Brawley.
The hot brine beneath the Salton Sea is very rich in dissolved minerals; in fact, there are so many minerals that they must be carefully managed to avoid damaging pipes and machinery in the geothermal facilities. Scientists think the origin of Salton Sea geothermal brine was partially evaporated Colorado River water from the Pleistocene era (2.58 million to 11,700 years ago) that flowed deep into the earth through fault lines (Williams and McKibben, 1989). The salty water heated up as it made its way deeper under the Earth’s surface, causing it to dissolve more minerals out of the underground rocks and turn into a complex solution loaded with metals (McKibben and Hardie, 1997; Garrett, 2004).
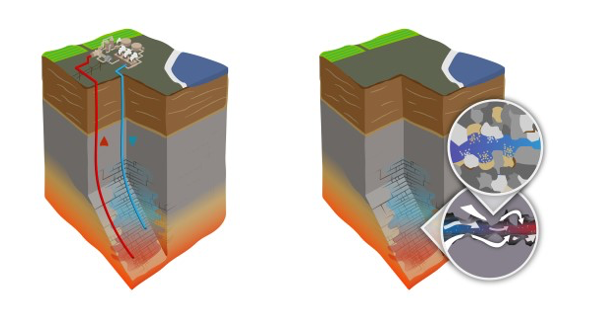
Depiction of the underground geothermal reservoir. The image on the left shows the hot brine coming up to the surface to supply the geothermal power plant, then being reinjected. The image on the right illustrates minerals from hard rock dissolving into the brine, and the brine heating up as it flows. Credit: Jeremy Snyder, Berkeley Lab
Our team estimates there are 0.76 million metric tons (MT) of lithium metal in the part of the reservoir that has already been developed for energy production (“proven reserves”), and as much as 2.6 million metric tons that are accessible without further evaporation of the Salton Sea or technological advancement (“accessible reserves). It’s estimated that about 24,000 MT of lithium flow through the existing geothermal plants each year. The maximum amount that could actually be produced is lower, because technology won’t be able to recover 100% of the lithium. Assuming a recovery rate of 90% (which is what the companies have stated their technology can achieve), there are 21,600 MT per year of recoverable lithium already flowing through the power plants. The table below calculates how many EVs could be produced with these amounts.[1]
| MT Lithium | MT LCE | # Tesla Model S | # Fiat 500s | # E-Bikes |
Recoverable in power plants today | 21,600 | 115,000 | 2.5 million | 5.1 million | 288 million |
Proven reserves | .76 million | 4.1 million | 90 million | 183 million | 10 billion |
Accessible reserves | 2.6 million | 13.7 million | 300 million | 613 million | 34 billion |
[1] Same assumptions as before: 85 kWh Tesla battery, 42 kWh Fiat 500 battery, 0.75 kWh e-bike battery, with composition of .1kg Li/kWh.
At this time, we can’t predict with certainty how many years of production the resource could support. The total amount that will be produced is partially determined by economics: the reservoir will get diluted over time, so at a certain point, the concentration of lithium will likely get low enough that it won’t make economic sense to extract it anymore. Exactly when this happens will depend on global factors like the how the price of lithium changes or the cost of extraction technology in the future, as well as local decisions, like how many extraction facilities are built and where the reinjection wells are located. In the next phase of our study, one of our goals is to improve our models of the reservoir and our economic analysis so we can make better predictions about how long production might last.
About Direct Lithium Extraction (DLE)
First, the operators have to remove other minerals from the brine to make it easier to recover lithium. To do this, they add inputs like limestone that react with interfering minerals to turn them into solids, which then sink to the bottom. This is called “precipitation” and the solids are called “precipitate.” The clarified brine then moves on to the lithium recovery circuit.
The companies are each developing their own technology to separate lithium from brine, and there are different processes that could be used. The most advanced process is called “adsorption,” which basically means “making one thing stick to another thing.” They’ll run the brine through columns filled with small beads composed of aluminum or titanium oxide particles, which selectively stick to dissolved Li and remove it from the brine. These beads are then washed and may be run through pool acid to strip out the lithium and other metallic ions and prepare the beads to be used again. After that, the remaining brine is reinjected back into the geothermal reservoir.
(References: Featherstone et al., 2020; Paranthaman et al., 2017; Stringfellow & Dobson, 2021; Vera et al., 2023.)
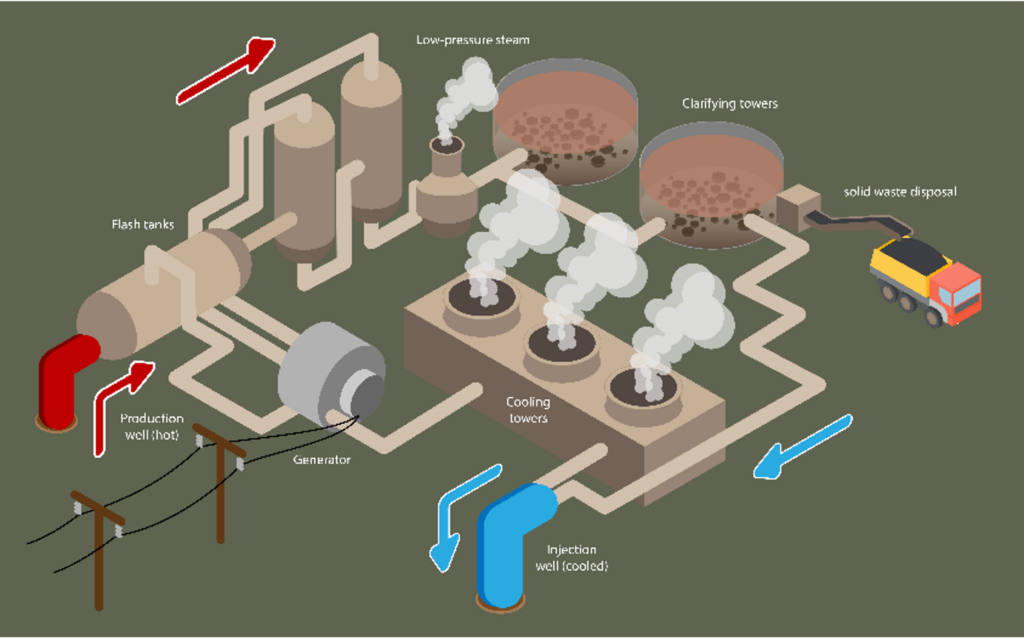
Illustration of a geothermal power plant, including the solids removal process (they settle to the bottom of the “clarifying towers”)
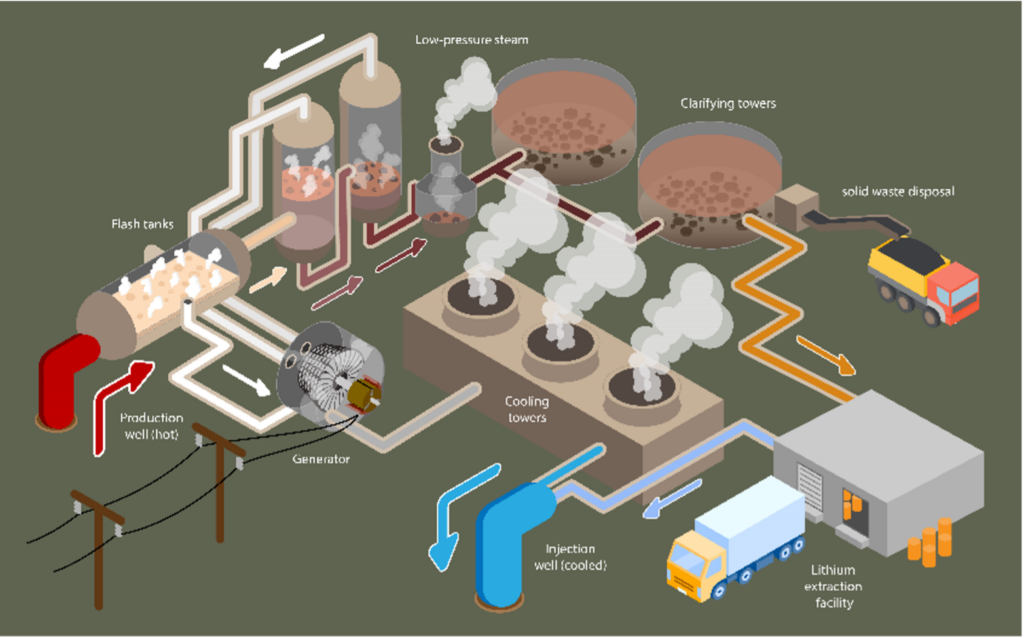
Geothermal power plant with a lithium extraction step added after the brine is clarified.
Yes. It’s mainly a question of whether the market value of the minerals is higher than the cost of recovering and purifying them. Apart from lithium, developers are most interested in recovering zinc and manganese. Manganese is another mineral that’s used in battery production, and it’s also used to make stainless steel. Zinc has a wide variety of uses, including galvanizing steel and iron.
After it’s extracted, the lithium is recovered as a salt called lithium chloride. To be used in a battery, lithium chloride has to be refined into a battery-grade compound: either lithium carbonate or lithium hydroxide monohydrate. Lithium carbonate is made by adding sodium carbonate (“soda ash”) to lithium chloride, and then the product is washed and dried to purify it to battery-grade quality. Producing lithium hydroxide requires additional processing: the lithium carbonate is combined with calcium hydroxide (also known as hydrated lime), and then purified into battery-grade material.
The rest of the brine is reinjected back into the reservoir.
This is about using the lithium to manufacture batteries and even EVs locally, rather than simply exporting it to make batteries somewhere else. There are a few reasons people are interested in doing this. It would enable more value to be captured locally, since each added refining and production step generates additional revenue and employment. It would also eliminate unnecessary transportation, which would reduce the environmental impact (including the carbon footprint) of producing batteries and EVs. Finally, it would support national security goals by keeping the supply chain within the U.S.
What would it actually mean to turn Lithium Valley into a supply hub? Essentially, it would mean attracting companies that specialize in other steps of the battery supply chain to the region. This could include:
- Battery component manufacturing, especially to make cathodes (the part of the battery that contains the most lithium).
- Cell and pack production facilities. To make batteries, different components (including the cathode) are assembled into cells. The cells are then configured to make a battery pack, which is equipped with software, electronics, and a protective outer casing.
- Car factories to make EVs.
- Recycling facilities to recover materials from battery packs after they’ve reached the end of their useful lives.
Water Use
The source of lithium is an underground brine reservoir, which cannot be used for drinking or irrigation. The brine is reinjected back into the reservoir after extraction. Nonetheless, geothermal energy and direct lithium extraction (DLE) do consume freshwater for some of their operations. In this section, we talk about water use for geothermal and lithium production, including the source of water and how much water the facilities are expected to consume.
Our technical report on water use can be accessed here. It includes an analysis of current water availability, projected water demand for expanded geothermal and lithium production, and the potential impact on future water availability under drought scenarios.
On average, the existing geothermal power plants in the region consume about 16 AF of water each year per MW of electricity generating capacity. That’s roughly equivalent to one Olympic swimming pool per year to power around 100 homes (CAISO, n.d.).[1] So a 50 MW plant, which is typical in this region, would consume around 800 AF per year, roughly the amount of water it would take to grow 130 acres of alfalfa (Montazar, n.d.).[2] The total consumption for all power plants in the Salton Sea Geothermal Field is approximately 6,500 AF/year, which is 0.25% of the total water supply for Imperial Irrigation District (IID).[3]
It’s more difficult to estimate water consumption for lithium extraction, because we don’t have enough data about the processes that will be used and how the technology will perform at a commercial scale. Transparent and ongoing reporting from demonstration facilities will be necessary so we can understand the implications for water consumption before the technology scales up. However, we do know that EnergySource Minerals’ allocation of water from IID is 3,400 AF per year for a commercial facility producing ~17,000 metric tons of LCE, which translates to ~0.2 AF per metric ton of LCE (although representatives from EnergySource Minerals stated in a public meeting they expect their actual water consumption will be less).
(References: Draft Environmental Impact Report for the Energy Source Mineral ATLiS Project, Imperial, California, 2021; Imperial Integrated Regional Water Management Plan.)
[1] 16 AF = 29,736 m3 = 7.9 swimming pools each year per MW of capacity – enough for 750 homes, according to the California Independent System Operator (CAISO). See this “Understanding Electricity” page from CAISO.
[2] One acre of alfalfa in Imperial County consumes 6-6.5 AF/year.
[3] IID’s total supply of water for 2020 was 2.6 million AF. See these slides from IID.
These processes will use canal water from the Colorado River that is supplied by IID for the ~0.2 AF needed per metric ton of LCE. To make the water stretch further, the facilities also plan to recover water from the geothermal steam once it has been condensed back into liquid (“steam condensate”) as an additional water supply at their facilities.
IID has rights to a specified amount of water from the canal each year, and they allocate how much of it can be used by agriculture, towns, and industrial facilities inside its service area. That means that a certain amount of water is set aside for industrial uses, like geothermal power production or lithium extraction. As of 2023, 97% of IID’s water allocation is used for agriculture. Drinking water and other household uses are classified as municipal water use, which is about 1.5% of IID’s allocation. Geothermal and other industrial uses are around 1%. At present, IID has set aside 25,000 AF of industrial water per year that can be used for lithium extraction and other industrial facilities. Based on the water allocation assumed by EnergySource Minerals, that would be enough to produce at least 125,000 metric tons of LCE per year.
If the region’s geothermal power production expands to 920 MW in coming years as planned, we estimate that ~15,000 AF of water will be needed annually for these power plants. IID’s future planning documents have allocated water for renewable energy in the region, including geothermal energy production. Extracting the lithium at geothermal power facilities producing 920 MW would require about 50,000 to 65,000 AF of water, depending on the extraction processes that will be used. Together, the planned geothermal and lithium production would represent about 6% of IID’s current allocation of Colorado River water.
The looming question is how much Colorado River water will be available in the coming decades, as the Colorado River Basin faces ongoing drought and long-term aridification. In May 2023, California, Nevada, and Arizona agreed to reduce water usage by about 14% between now and 2026. In 2026, water allocations will be renegotiated. Under scenarios with low future water supply, water would need to be reallocated from agriculture to meet demand for planned lithium and geothermal expansion (although agriculture would still be by far the largest user in the region). To learn more about proposed Colorado River allocation cuts, see this article from CalMatters.
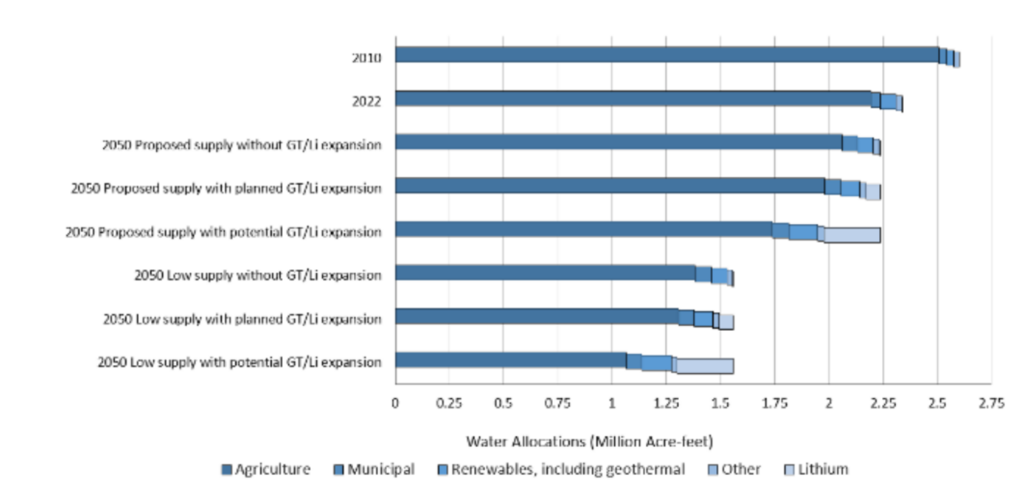
Estimate of IID water required for expanded geothermal energy production and lithium extraction processes under different Colorado River water supply scenarios.
Geothermal power plants use water primarily:
- In cooling towers. After electricity is generated, cooling towers circulate cool water to lower the temperature of steam so it turns it back into a liquid and can be reinjected underground. Some of this water is reused for other operations, but some is lost to the atmosphere through evaporation.
- To dilute the brine. If the brine is too salty, it interferes with the plants’ ability to generate electricity. Water consumption by SSKGRA power plants varies because power plants with the saltiest brine have to use more water to dilute it.
For adsorption-based lithium extraction, water will likely be used to strip lithium chloride compounds away from the sorbent once they have been separated out of the brine, and to wash hydrochloric acid from the sorbent beads. Again, it’s difficult to say for sure without concrete data about the extraction technology.
All companies involved in lithium extraction have stated plans to recycle water in their processes. However, we don’t have data about what percentage of water will be recycled, how many times water can be recycled, and whether they have already accounted for recycling in their water use estimates. As a result, water recycling was not part of this study.
No. The facilities can only use water that is allocated for industrial use, so municipal supplies should be unaffected. Neither the geothermal nor lithium extraction processes are expected to interfere with water quality. Companies are required to treat process water onsite before it is discharged.
None of the facilities will use water from the Salton Sea or affect Salton Sea water levels through any of their operations.
The main factor that has historically affected Salton Sea levels is the amount of water flowing in from surrounding farms. When water conservation measures are put in place, there is less agricultural runoff, which causes the water level of the lake to drop. So if continued drought means that IID has less Colorado River water to allocate, farms would likely be required to reduce their water consumption, which would affect Salton Sea water level.
Environmental Impacts
If you have driven near the geothermal power plants near the southern end of the Salton Sea, you have likely noticed plumes of vapor rising from the stacks. This vapor is over 99% water and 0.2% carbon dioxide (CO2), with trace amounts of hydrogen sulfide (H2S) and ammonia (NH3).
The power plants near the Salton Sea each emit less than 25,000 tons of CO2 per year. Relative to the energy they produce, this is six times lower than emissions from a natural gas power plant, and 13 times lower than a coal-fired plant. For coal and natural gas power plants, most emissions are released when the fuel is burned (combusted) to generate heat to make steam. Geothermal energy doesn’t use combustion, so any emissions are from molecules in the brine that escape into air as the pressure dissipates at the surface. In the future, some CO2 emissions from brine and steam might be captured and used to make lithium carbonate.
The H2S emitted by geothermal power plants is approximately one-tenth the amount of H2S that is naturally emitted by the Salton Sea. Power plants in the SSKGRA use a variety of methods to control H2S emissions. The most common approach is to transform it into a form that is not released into the atmosphere.
It’s harder to estimate emissions from the DLE process specifically, because again, companies are still developing their technologies. The emissions from DLE are not expected to be significant since the process will not use combustion, but without knowing exactly what chemicals and mitigation technologies will be used, we can’t give an exact estimate. Each company will begin with a small demonstration facility before they build a commercial-scale factory, and they will have to demonstrate that they meet local air quality standards to obtain a permit.
More information and resources about air emissions:
- The technical report’s chapter about air emissions starts on page 100 of the full report.
- For a more detailed description of H2S and H2S abatement technology, see pages 114-117 and 128-129 of the full report.
- Power plants operating in California are required to mitigate these emissions and report them to the California Air Resources Board (CARB) if they are above a certain threshold. To access data about GHG emissions from geothermal facilities (and others), look here.
- Tip: The data is in a spreadsheet. If you click on “2021 GHG Facility and Entity Emissions,” it will download an Excel file.
- To access data about criteria and toxics pollutant emissions, look here.
- Tip: Click on “Begin your facility search here,” which will open a search engine. Select Imperial County from the “district” dropdown menu and hit submit to look at facilities in Imperial County.
The main solid byproduct of geothermal and lithium production is iron-rich silica precipitate, which has a similar texture to sand and usually isn’t hazardous. Geothermal operators already have to remove silica from the brine before it can be reinjected; otherwise it would build up in pipes and damage the machinery. Operators will need to remove more silica to purify the brine before they can extract lithium. The silica precipitate, known as “filter cake,” is currently sent to a landfill (more details on this in the next question), but companies are actively looking for buyers who can use filter cake to make products such as silica sand for cement. And again, the amount of waste that’s generated will depend on the lithium extraction technology and whether the byproducts can be used for other purposes, instead of getting landfilled.
A high-end estimate of the additional solid waste that could result if all lithium were extracted from today’s processed brine would be 889,000 tons/year (although it should be less, based on our calculations). That would be approximately 85 truckloads per day across the SS-KGRA, and a little over 10 trucks per power plant.[1] For reference, average daily travel on Highway 11 near Niland is approximately 3,500 vehicles per day (CalTrans, 2019).
The waste is mainly silica, but there are also trace quantities of arsenic, lead, and other minerals that must be handled carefully. Approximately one-fifth to one-third of geothermal power plant solid wastes require management as hazardous wastes under California regulations.
[1] Assuming each truck carries 28 tons.
First, solid waste must be tested for hazardous materials to determine which landfill it should be sent to. The destination of the waste depends on the company and whether it meets hazardous waste thresholds, which are stricter in California compared to federal standards. For nonhazardous waste, Berkshire Hathaway Energy Renewables (BHER) operates its own monofill (a landfill for only one type of waste) outside Brawley, while EnergySource, a smaller company, uses the Salton City landfill. Waste that exceeds the California threshold but is nonhazardous by federal standards is sent to an industrial landfill in Yuma, Arizona. Waste that is deemed hazardous by both state and federal standards is sent to the Button Willow landfill in Kern County, which is permitted to handle hazardous waste.
Companies are required to report data about their hazardous waste generation to the California Department of Toxic Substance Control; you can access that information on DTSC’s website.

Waste storage at BHER facility.
Credit: Jeremy Snyder/Berkeley Lab
The Salton Sea Geothermal Field (SSGF) is in a tectonically and volcanically active area; this is actually what makes the brine hot enough to generate electricity. Our team looked at historic data to better understand the relationship between geothermal energy production and local seismicity rates, which reflect the frequency of earthquakes. It’s important to note that data measuring seismicity is limited; a detailed seismicity catalog is only available from 1972 onward, which makes it difficult to know if recent activity is part of a larger pattern, or attribute changes to geothermal production with certainty.
Our analysis found that seismic activity in the SSGF was lower than in the broader seismic zone in the 1970s but increased after geothermal energy started being produced in 1982. However, seismicity rates leveled out in the early 2000s, and since 1996 they have been weakly correlated with the volume of fluid being injected by the power plants. Seismicity rates in the SSGF are now similar to the overall Brawley Seismic Zone, which is controlled by tectonic activity.
Lithium extraction is not expected to have an impact on seismicity if the production/injection rate stays at the current level, because extraction happens after brine is brought to the surface and does not require any additional drilling or reinjection. However, it is important that operators take precautions and avoid siting injection wells near active faults when they construct new geothermal plants.
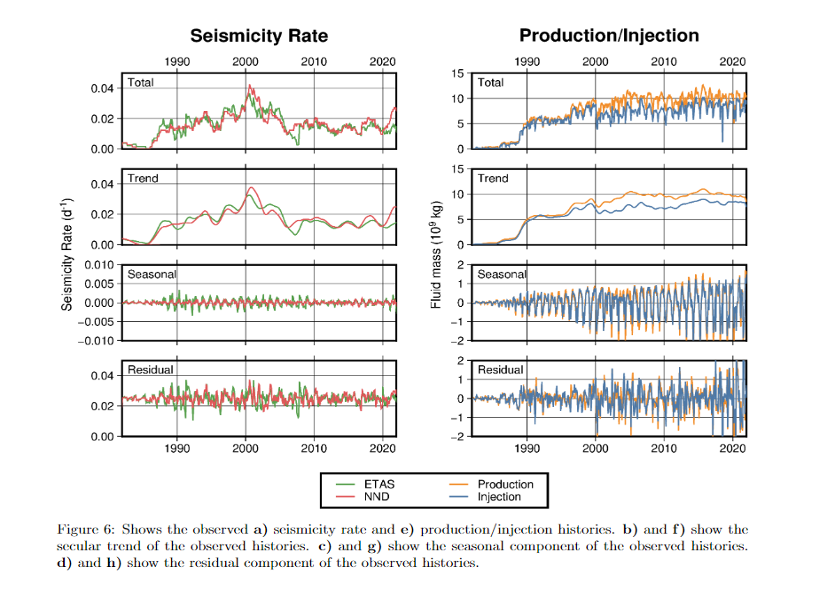
Recorded seismicity rates next to the volume of fluid being produced and injected into the geothermal reservoir from the chapter on seismicity. The technical report’s chapter on seismicity starts on page 159.
Geothermal power plants use chemicals to prevent minerals from building up inside the machinery and to control air pollutant emissions. Plants have to report which chemicals they use to the Regional Water Quality Control Board and submit safety data sheets to get approval to operate. The chemicals used in power plants at the Salton Sea Geothermal Field include phosphate-based corrosion inhibitors and “TowerBrom,” a product used to mitigate hydrogen sulfide emissions. These are similar to chemicals used for water treatment and process control at other industrial facilities.
For more information, see the chapter on chemical use and solid waste, which starts on page 126 of the full report.
With DLE still under development, there are currently two main ways of producing lithium: hard rock mining and brine evaporation. In hard rock mining, typically a substance called spodumene ore is physically extracted, heated, pulverized, mixed with acid, re-heated, re-filtered and concentrated to form lithium carbonate (though the exact process varies depending on the characteristics of the ore). This is an expensive and energy-intensive process that uses fossil-fuel generated heat and electricity. With brine evaporation ponds, lithium-rich brine is pumped to the surface in hot, dry areas. The ponds essentially let brine sit in the sun for 1-2 years, which evaporates some of the water, leaving behind a more concentrated solution that can be processed into battery-grade materials. Brine evaporation is less energy intensive, and its freshwater consumption is low compared to other forms of lithium extraction. However, evaporation uses vast expanses of land – up to 3,000 acres, compared to just 50 acres for geothermal DLE. Substantial amounts of brine must be pumped to the surface, which requires energy, and much of this brine is then lost through evaporation.DLE uses the least land of the three methods, and it has lower carbon emissions than ore-based mining. Our analysis finds that the water consumption of DLE is lower than evaporation if you count the water from brine, but slightly higher than estimates from ore-based mining (although this is based on water allocations that may be higher than the actual process consumption).
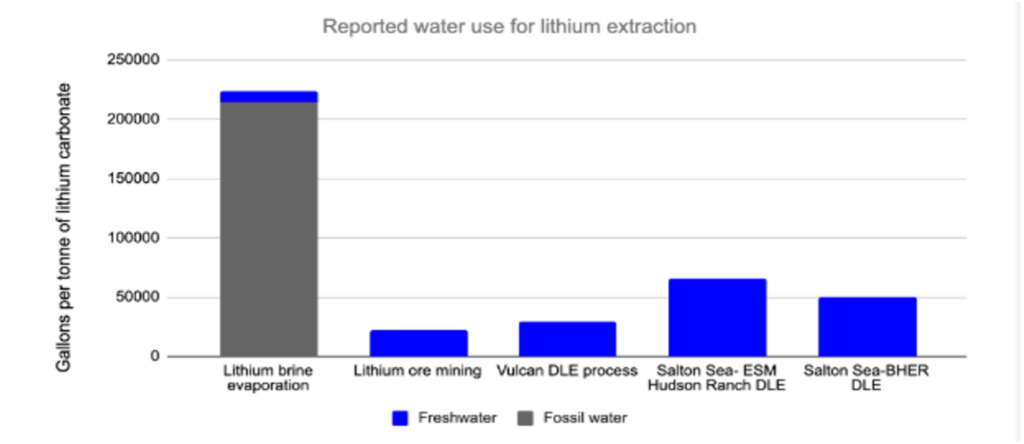
Figure comparing the water usage for different lithium extraction methods from our technical report. “DLE” is direct lithium extraction. “ESM” is Energy Source Materials, and “BHER” is Berkshire Hathaway Energy Renewables
The following references have more information on the environmental impacts of different lithium extraction processes: Dai et al., 2019; Flexer et al., 2018; Kelly et al., 2019, 2021; Schenker et al., 2022; Vera et al., 2023; Vulcan Energy, 2021.
Out-of-scope Questions
Public health is top-of-mind for communities everywhere, and especially for communities near the Salton Sea who face extreme challenges related to asthma and air quality. For any development to occur in the area, it is crucial to consider potential impacts on public health and how public health will be monitored and protected. While our study did not surface any red flags, we are not able to address questions about public health. We compiled preliminary data about emissions and waste streams, but further research involving both public health experts and active community participation would be necessary to provide more concrete information.
The properties of lithium itself and its potential health implications were also outside the scope of this report. Looking at other studies, we did not find evidence suggesting that it is unsafe to be around the compounds that will be produced near the Salton Sea (i.e., lithium chloride, lithium carbonate, and lithium hydroxide), but public health has not been extensively studied in the context of battery production. At this time, most information about lithium and health comes from studies about using lithium to treat bipolar disorder, a psychological condition. Patients taking lithium as treatment for bipolar disorder must be careful to follow doctor’s instructions to avoid lithium toxicity, which can occur when people take too much lithium medication, or if they are taking other medications that cause an interaction. The symptoms of lithium intoxication range from nausea to seizures at extremely high concentrations (>3.5mEq/L) (Hedya et al., 2023). The European Chemicals Agency considered classifying lithium as hazardous for reproduction based on studies about reproductive health outcomes for medical patients taking very high doses of lithium. The Agency concluded there was not cause to classify lithium as hazardous for cancer or cell mutation (Bjorge & Husa, 2021).
Another known safety hazard associated with lithium is related to lithium-ion batteries. Lithium-ion batteries must be handled and stored carefully because if they are physically damaged or catch fire, a chemical reaction can be triggered that produces more and more heat, called “thermal runaway.” These batteries are not more likely to catch fire than regular car batteries, but the possibility of thermal runaway makes lithium-ion battery fires more dangerous and complicated to put out if an incident does occur because they reach such high temperatures (Kong et al., 2018; National Transportation Safety Board, 2020).
The quality and quantity of jobs that could be created by expanding geothermal and lithium production is certainly a Frequently Asked Question, along with what training will be available so local residents are eligible for those jobs. However, we do not address socioeconomic topics like workforce development in our report because our team’s expertise is in physical sciences.
Companies have provided job estimates on their websites and in different presentations, however. For example, Energy Source Minerals has stated they plan to hire 70 employees for their lithium-related operations. Several local institutions are involved in training or advocacy related to workforce development. Imperial Valley College is currently developing programs to prepare students for careers in the geothermal and lithium industry, with a plant operator certification program starting in fall 2023. San Diego State University has received $80 million to build a science, technology, engineering and math (STEM) campus nearby, in Brawley. Several labor unions have also been involved in advocacy surrounding Lithium Valley, including IBEW-569; San Diego & Imperial Counties Labor Council, AFL-CIO; United Auto Workers, Western States; and UA Local 230 (Partners, n.d.).
The State of California passed SB 125, a lithium tax bill, in 2022 that assesses up to $800/ton of LCE produced. How this revenue is invested will be a factor in determining whether and how surrounding communities benefit from a potential lithium industry. However, it is not appropriate for our team to speak to this issue as again, it is outside our area of expertise, and we are not from the area.
SB 125 also allocated $5 million to Imperial County to prepare a programmatic environmental impact report, support community outreach and stakeholder engagement, and gather public input. At the time of writing, this process is ongoing, with outreach supported by community-based organizations in the area. The county maintains a website with information about Lithium Valley, including announcements about upcoming workshops and a document containing all public comments submitted about revenue investment.
The table below contains website addresses for some of the community-based organizations who received SB 125 funding for Lithium Valley outreach.
Organization | Website |
Comite Civico del Valle | |
Imperial Valley Equity and Justice Coalition | |
Los Amigos de la Comunidad IV | |
Imperial Valley LGBT Resource Center |
According to SB 125, 20% of the tax revenue from lithium should be allocated to Salton Sea restoration. You can learn more about its Salton Sea restoration projects here.
References
Bjorge, C., & Husa, S. (2021). Opinion proposing harmonised classification and labelling at EU level of lithium carbonate [1] lithium chloride [2] lithium hydroxide [3] (No. CLH-O-0000007034-82-01/F). European Chemicals Agency. https://echa.europa.eu/documents/10162/e2a3c38e-85fe-505c-a325-293c70a74da5
CAISO. (n.d.). Understanding electricity. California ISO. Retrieved July 31, 2023, from http://www.caiso.com/about/Pages/OurBusiness/Understanding-electricity.aspx
CalTrans. (2019). Traffic Census Program. CA.gov. https://dot.ca.gov/programs/traffic-operations/census
Draft Environmental Impact Report for the Energy Source Mineral ATLiS Project, Imperial, California. (2021). Chambers Group, Inc. . https://files.ceqanet.opr.ca.gov/266414-3/attachment/1WAuc8St0q6pOKUU198nvMAjVyToYppAVahm_j0pGKvijQDseTzebhSqUWzg_s9AsKTPbLRQ_XPG7h4b0
Featherstone, J. L., Hanson, P. J., Garska, M. J., & Marston, C. R. (2020). Process for Recovery of Lithium from a Geothermal Brine (U.S. Patent Office Patent No. 10604414). In Patent (No. 10604414). https://patents.justia.com/patent/20200189925
Garrett, D. E. (2004). Handbook of Lithium and Natural Calcium Chloride. Elsevier.
Godwin, L. H., Haigler, L. B., Rioux, R. L., White, D. E., Muffler, L. J. P., & Wayland, R. G. (1971). Classification of Public lands Valuable for Geothermal Steam and Associated Geothermal Resources. Geological Survey Circular, 647. https://pubs.usgs.gov/circ/1971/0647/report.pdf
Hedya, S. A., Avula, A., & Swoboda, H. D. (2023). Lithium Toxicity. StatPearls Publishing.
Imperial Integrated Regional Water Management Plan. (2012). Imperial Irrigation District. https://www.iid.com/home/showpublisheddocument/9571/635648001335730000
Jaskula, B. W. (2023). Lithium Data Sheet – Mineral Commodity Summaries 2023. USGS. https://pubs.usgs.gov/periodicals/mcs2023/mcs2023-lithium.pdf
Kelly, J. C., Dai, Q., & Wang, M. (2019). Globally regional life cycle analysis of automotive lithium-ion nickel manganese cobalt batteries. Mitigation and Adaptation Strategies for Global Change. https://doi.org/10.1007/s11027-019-09869-2
Lithium Valley. (2022, February 2). Imperial County; Imperial County. https://lithiumvalley.imperialcounty.org/
Montazar, A. (n.d.). Water Quality FAQs. University of California Agriculture and Natural Resources. Retrieved February 13, 2023, from https://ceimperial.ucanr.edu/Custom_Program275/Water_Quality_FAQs/
National Transportation Safety Board. (2020). Safety Risks to Emergency Responders from Lithium-Ion Battery Fires in Electric Vehicles (No. NTSB/SR-20/01). NTSB. https://www.ntsb.gov/safety/safety-studies/Documents/SR2001.pdf
Partners. (n.d.). Lithium Valley Community Coalition. Retrieved August 4, 2023, from https://www.lithiumvalleycommunitycoalition.org/partners
SB-125 Public resources: geothermal resources: lithium, SB 125, California State Senate, 2022. Retrieved June 27, 2023, from https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=202120220SB125
Vulcan Energy. (2021). 2021 Sustainability Report. https://annualreport.v-er.eu/wp-content/uploads/Vulcan-Energy-Sustainability-Report.pdf
